Charles L. Coltman IV, Ignatius Beard, and Scott A. Irwin, MD
Background
Collecting patient-reported outcomes have been shown to improve outcomes in patients with cancer; however, implementing methods to collect and utilize these data, such as remote patient monitoring, has been limited due to staffing resources, workflow disruption, EMR integration, and cost barriers. A self-care patient engagement consumer app is one solution to help alleviate these barriers. The purpose of this study was to demonstrate that the self-reporting of symptoms by patients using a novel patient engagement mobile application, at any time, from anywhere, would have a meaningful impact on symptom burden while improving Quality of Life (QoL) without the aforementioned barriers, most importantly workflow disruption.
Design
A blinded, two-arm, randomized, controlled trial of CancerLife™ was conducted. Participants with breast cancer were recruited nationwide via Facebook ads and asked to complete an online qualification survey. If qualified, they received a text message with a link to complete the consent and enrollment process, then randomly assigned via 1:3 ratio to control or the CancerLife™ intervention. Overall health state (EQ-5D) and QoL (EQ-VAS) were compared with usual care every three weeks from 9 weeks to 24 weeks post-baseline. Virtual monitoring of common cancer-related symptoms (symptom count) was also done for the CancerLife™ group, with participants asked to share their app-generated printed reports w their care team at their consultation appointment.
Results
A total of 1006 participants were recruited online, and 499 completed the registration, consenting process, and download app procedure and were enrolled. A total of 189 participants in both groups completed the 24-week study. Participants that enrolled represented a wide national geographic area inclusive of 117 different area codes, which suggests the solution can address the challenges of care disparities.
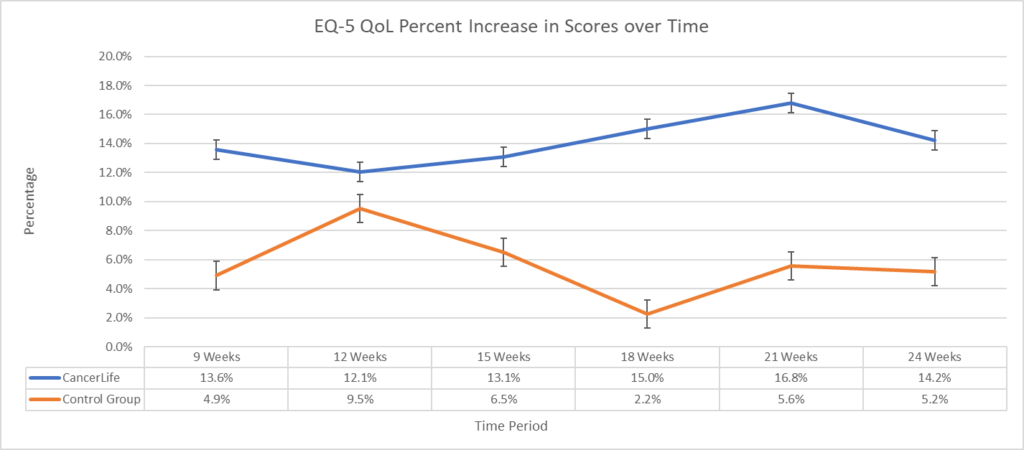
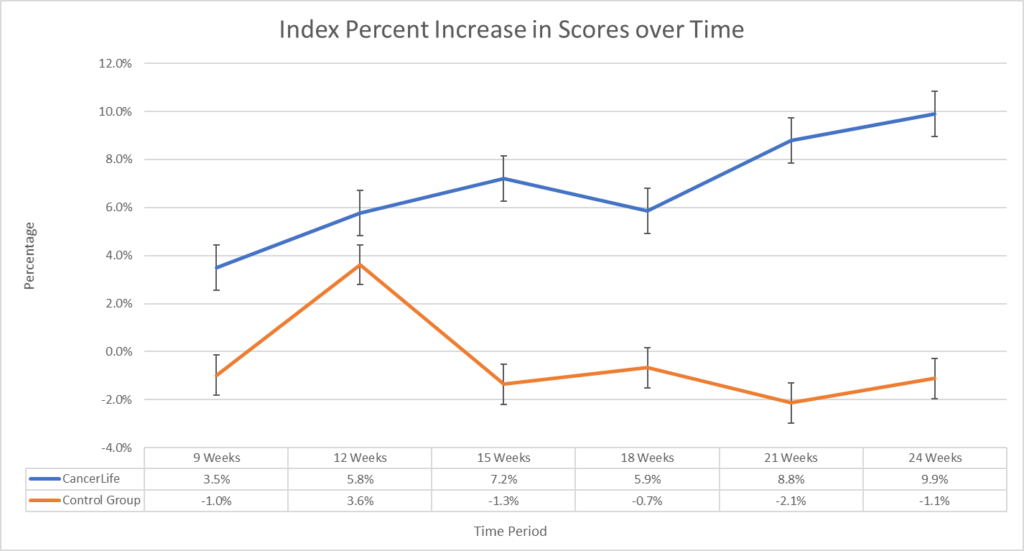
A significant main improvement effect of intervention favoring CancerLife™ was found for both the EQ-VAS (F(1, 5) = 22.1, p < 0.01) of 14.2% and 9.9% on the EQ5-D (F(1, 5) = 14.3, p < 0.01). Post-hoc paired t-test comparisons indicated significantly higher mean differences at 18 and 21 weeks (p < 0.05) on the EQ-VAS and at 21 weeks (p < 0.05) on the EQ5-D, with similar trends at 9, 15, and 24 weeks on the EQ-VAS and 15, 18, and 24 weeks on the EQ5-D. Further, the CancerLife™ group demonstrated a significant decrease in symptom count (mean Δ=-9.11, -78.1%, t=-2.62, p<0.001) at 24 weeks.
Conclusions
This study demonstrates that a patient engagement app with a novel data collection platform could lower symptom burden and improve overall QoL with minimal barriers to implementation. Since remote patient monitoring systems are hard to implement inside cancer care settings due to costs, IT system integration, and workflow disruption, a direct-to-consumer-based app, which can be used in any care setting by any patient, and accessed through any connected device, shows significant promise to ameliorate symptom burden, raise overall QoL, and address disparities of access to care and care outcomes for cancer patients. Providers may consider this as a tool to improve their population quality metrics, care and care utilization outcomes, disparities, and patient satisfaction scores without significant startup, maintenance, resource, and workflow costs.