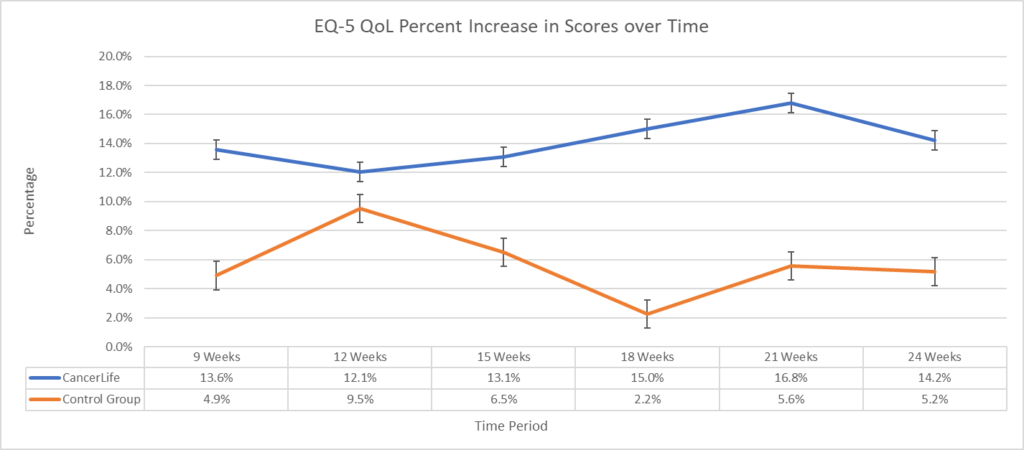
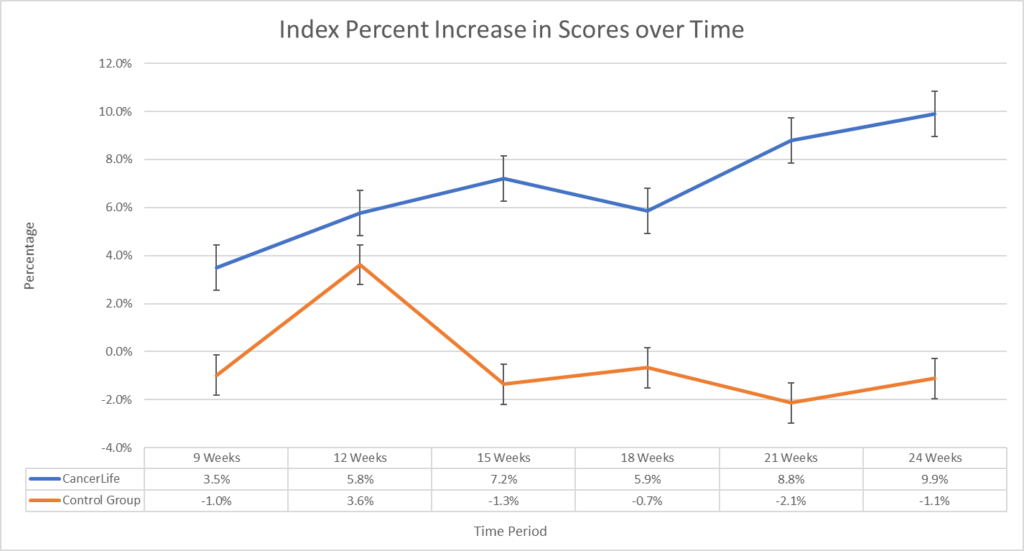
What are 5 FREE Patient Support Services that are rarely used or known about?
Hi, Charlie Copeman here, CEO o and founder of Cancer Life. I just thought this,
Today’s blog, we would talk about Five underutilized and underappreciated services that patient support services that cancer patients have access
And are available that sometimes that cancer patients aren’t aware of. So the first one is, if you’re going through cancer, your journey, one of the biggest challenges with mental Health. Obviously the nature of the fear The anxiety, the worry
Can have a dramatic impact on your mental health and how do you manage that. There’s, from some statistics, 50% of patients experience some form of depression during their journey. That’s completely un understandable and rare. Rarely of those, only 10 to 20%
Actually get treatment or seek out Help for tho those those needs.
Folks need to understand that if you’re in a, some type of cancer center, you know, typically, they do have social workers that are available that you can schedule time with to go over. I wouldn’t call them necessarily getting a psychiatrist, but those services are where they can help you. One, navigate, you know, your decision-making, navigate your relationships, which is a huge stressor for cancer patients.
And you really can get the support you need from folks that really understand how it works, what they’ve been through, you know, these folks have seen it all. So, number one social worker, social support services inside of cancer centers is really an untapped area to which you can seek help. Number two is inside those cancer centers. If you’re having financial stresses around getting to and from your appointments or any of the other, you know, financial burdens associated with your treatment, talk to the cancer center and ask to see if they have assistance programs. So a lot of them do, right? There’s a lot. Sometimes it’s, it’s through their nonprofit, you know, or there are other partners like the American Cancer Society that they partner with that has access to some of those services. Third, if you’re in a clinical trial in terms of financial services and support, look to those clinical trial partners.
The sponsors of those partners typically do have almost like a debit card that you can, that they give you to handle secondary expenses. That’s a good area where you can seek support. So if you’re in a clinical trial or planning to be ask about patient support services associated with that clinical trial, like I said, they’ll give you a debit card, you know, I don’t know, a thousand dollars a month, $500 a month to handle those secondary expenses. Also if you’re having trouble paying for medications, you know, doctors really don’t make treatment decisions around, you know, what’s the cost to the actual patient. They don’t know what your insurance is and what your, what the, what they’ll cover and what they’re not covered. They don’t know what your copay is. But if you’re under financial burden and your doctor has switched your medication to some newly approved drug or some other, you know treatment that’s highly specialized, which means it’s more money talk to the pharmaceutical company that your drug that is manufactured by the drug, talk to the manufacturer.
They’re called patient support services Manufacturers. Give away billions of dollars in free drugs every year. There’s a company called the Lash Group in which you can log onto their website, fill out some information, and you can sign up for the, get your drugs for free. Essentially, it’s a real, frankly, a pain in the to go through the process. They’re gonna call you a million times to see if you’ve taken your medication. They’re gonna ask you a million research questions, but, you know, in terms of getting something for free it’s something that, you know, patients need to learn about. So go to your specific, specific pharmaceutical company and you know, log onto their website and, and find out if they have patient assistance programs. And the final one, I just would like to say in terms of nutrition, this is a big area that’s happening now, is that, that the cancer centers are providing nutrition services through partners.
You know, at the very least, a lot of the time there are nutrition shakes. So if you’re gaining, you’re having trouble keeping on weight, you’re having trouble eating because it’s, you get too nauseous, you know, they do have those nutrition shakes that I believe they partner with Nestle who provides these, these shakes. You can get those for free. So anyway, that’s, that’s five services. I think, you know, every cancer patient going through treatment in order to get support, you know, realize this, these are areas in which you can explore.
All right, so anyway, thank you so much.