Guest Blog Post : CancerLife, a New Cancer Support App that can raise Quality of Life for each member.
By Genna Gibbons – Patient Advocate
Cancerlife was founded by Charlie Coltman who lost his best friend to pancreatic cancer. He founded the company with a mission to improve the lives of cancer patients through the use of innovative technology. Its flagship product is a cancer support app that provides patients with various tools and resources to help them manage their condition and navigate the cancer journey with online social support network. This can be especially beneficial for cancer patients, who may have compromised immune systems and may be at higher risk of infection. Cancerlife can also help patients in rural or underserved areas who may not have access to specialized cancer care, connect with other support resources online.
The CancerLife app also includes a symptom collection and racking feature that allows patients to record and monitor their symptoms over time. This can be helpful for both patients and their healthcare providers, as it can provide valuable insight into the effectiveness of treatment and help identify any potential issues. Since roughly 50% of all symptom and side effect issues go unaddressed, which can degrade QoL, the Cancerlife app is directly addressing this huge problem. But most importantly, Cancerlife is a social network like Facebook which creates a powerful engagement experience for each user.
CancerlIfe has been conducting extensive research on how its platform measurably improves QoL. Recently it announce the results of its two-arm randomized control trial in which was conducted nationwide and enrolled 499 participants with breast cancer, aimed to evaluate the effectiveness of CancerLife’s platform in improving patient outcomes and experiences.
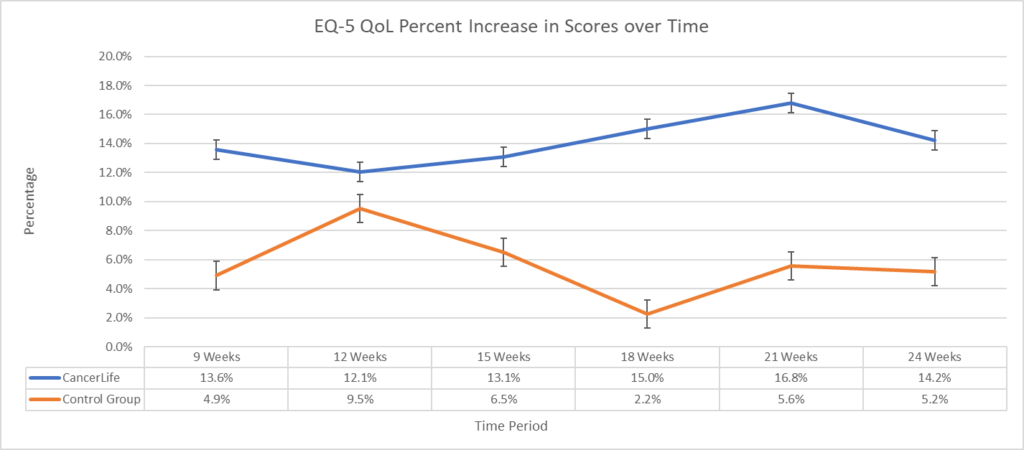
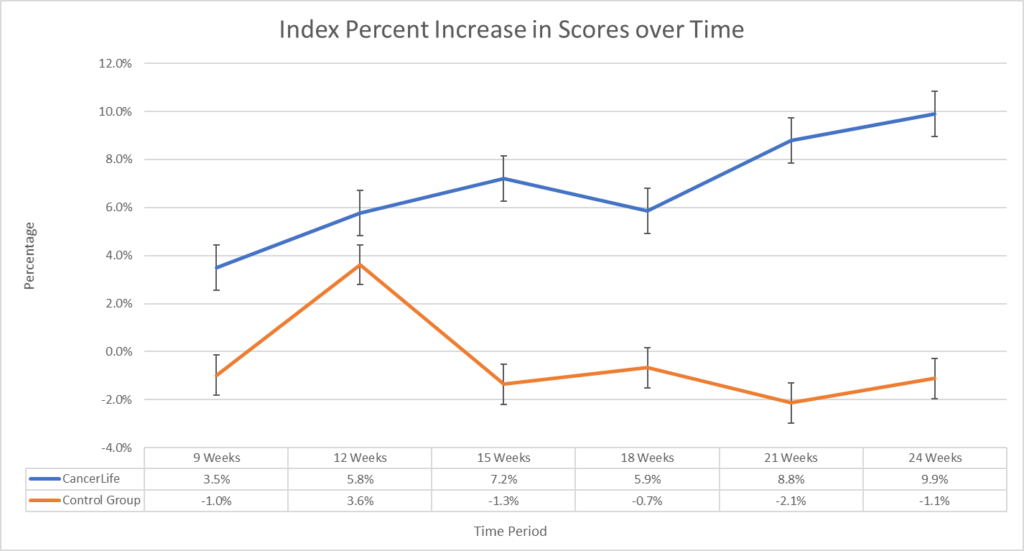
The trial enrolled participants via Facebook ads and randomly assigned them to either the intervention group or the control group. The intervention group received access to CancerLife’s platform, which includes a social support platform and novel symptom data collection tracking, and treatment planning tools. The control group received standard care. The results of the trial were highly encouraging. A significant main improvement effect of intervention favoring CancerLife™ was found for both the EQ-VAS (F(1, 5) = 22.1, p < 0.01) of 14.2% and 9.9% on the EQ5-D (F(1, 5) = 14.3, p < 0.01).
In addition to these individual benefits, the CancerLife app can also support the delivery of cancer care at a population level. Since Cancerlife does not need to be implemented inside a cancer center, it has the potential to scale into any care setting, including community oncologists, which represent 70% of the market. In their clinical trial results, patients were recruited from over 117 area codes which means the outcomes were achieved regardless of care location. CancerLife therefore can address disparities of care, so long as those patients have cell phone or internet access.
Despite the potential benefits of the CancerLife app, there are also challenges associated with its use. One concern is the issue of privacy and security, as patients may be reluctant to share sensitive personal and medical information online. There is also the potential for a digital divide, as not all patients may have access to the necessary technology or may not be comfortable using it.
To address these challenges, it is important that the CancerLife app is developed and implemented in a way that is sensitive to the needs and preferences of cancer patients. This may involve providing training and support to help patients feel comfortable using the app, as well as ensuring that appropriate security measures are in place to protect patient data.
Overall, the CancerLife app has the potential to greatly improve the way we support cancer patients, from improving access to care to empowering patients to better manage their condition. While there are challenges to be addressed, the benefits of this tool are clear and it is important that we continue to explore and invest in its development and use. In conclusion, the CancerLife app can greatly improve the lives of cancer patients and make the healthcare system more efficient. If Cancerlife can get the partnerships necessary to grow its users and scale into health systems, the platform could be a game changer to cancer care delivery. Cant wait to see what happens next!